Chu-Huang Chen ชคZ~i[ลu
utFChu-Huang Chen
Director, Vascular & Medicinal Research, Texas Heart Institute, USA
๚F฿aณN926๚iุj18F00`19F00
๊Fฎค@Cmu`บ
่FRole of the L5-LOX-1 Signalling Axis in Cardiovascular Aging
 @@
@@
ญ\ฬTvF
Atherosclerotic cardiovascular disease is a manifestation of cardiovascular aging, which progresses
at a different pace from one individual to another. Although
atherosclerosis can be initiated by a variety of factors, the common
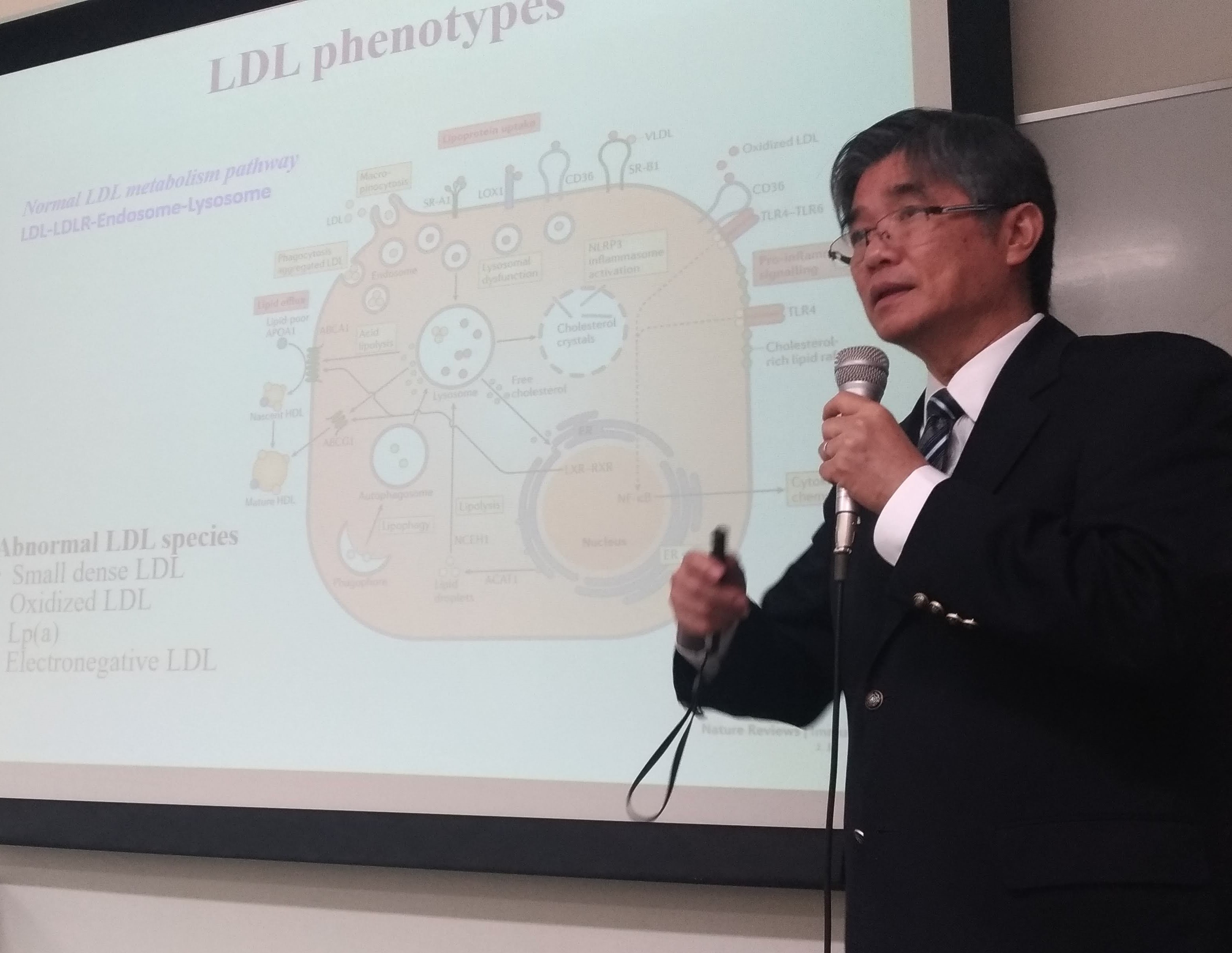
pathological denominator is deposition of low-density lipoprotein (LDL) in the
arterial walls. Because normal LDL is health beneficial, it has long
been speculated that the gculprith LDL subtype differs from normal LDL constitutionally. Both oxidized LDL and small-dense LDL are considered
atherogenic for a number of reasons but neither has been
isolated from circulation for chemical or functional scrutiny. In
contrast, electronegative LDL is easily retrievable from the plasma.
Through anion-exchange chromatography, human LDL can be divided
into 5 subfractions, L1-L5, with L5 being the most electronegative.
By comparison, L1 is the least electronegative subfraction, which
represents the majority of LDL
particles. Whereas L1 provides nutrition
to cells, L5 induces inflammation, senescence, and apoptosis
in a variety of vascular cells. Unlike L1, which is endocytosed
by normal LDL receptor (LDLR),L5 is rejected by LDLR. Instead, L5
signals through lectin-like oxidized LDL receptor-1 (LOX-1), which
was cloned against oxidized LDL epitopes by Dr. Tatsuya Sawamura and
colleagues in the early 1990fs. In addition to L5 and oxidized LDL,
LOX-1 mediates the signaling of many substances that are also negatively
charged, such as C-reactiveprotein (CRP) and tumor necrosis factor
alpha (TNF-ฟ). Regardless of its ligands, LOX-1 signaling induces a
spectrum of inflammatory changes in cells. Of importance, L5 is a
naturally-occurring lipoprotein that not only induces
expression of CRP and TNF-ฟ in endothelial cells but also interacts
with these cytokines to accentuate inflammatory reactions. In
human platelets, L5 acts via LOX-1 to enhance both the expression and
the effect of amyloid-beta totrigger platelet aggregation. Scarce in
healthy subjects, L5 is increased in patients with
cardiometabolic disorders and autoimmune states. In patients undergoing acute
ischemic events, such as acute myocardial infarction and acute ischemic
stroke, L5 is remarkably elevated in the circulation. Most
recently, we found that both LOX-1 and its soluble fragment, sLOX-1, are
increased in patients with ST-elevation myocardial infarction, in
concomitance with elevation of plasma L5. LOX-1 is minimally expressed
in vascular endothelial cells under normal conditions, but its
expression can be significantly augmented by L5. Mechanistically, L5
induces senescence of arterial endothelial cells by disrupting
mitochondrial function via LOX-1. In summary, the L5-LOX-1 signaling axis
plays an important role in cardiovascular aging and should be
considered a new treatment target in this formidable disease.

